Abstract
At sites of vessel injury, thrombin acts as the central mediator of coagulation through catalyzing fibrin clot formation and platelet activation. Currently, thrombin generation is measured using small molecule substrates. These substrates are limited in that they detect both active thrombin and thrombin bound to its inhibitor α2-macroglobulin (α2-M) and have limited utility in whole blood (Ninivaggi et al. Clin Chem 2012). In addition, these substrates contain short thrombin recognition sequences ranging from 3-4 amino acids and are unable to form additional exosite interactions with thrombin, which limit their specificity (Gallwitz et al. Plos One 2012). Furthermore, they are unfeasible for in vivo use because their hydrophobicity allows them to enter cells. However, plasma assays are limited because they ignore the hemostatic contributions of red blood cells, platelets and leukocytes and require anticoagulation and the addition of exogenous coagulation activators (Swystun & Liaw. Blood 2016 & do Amaral et al. Stem Cells Int 2016). Thus, we have developed a fluorescence resonance energy transfer (FRET)-based thrombin sensor to overcome these limitations.
We cloned and expressed the construct encoding the FRET-based thrombin substrate in Escherichia coli and purified the substrate using affinity chromatography. We confirmed using Sodium dodecyl sulfate−polyacrylamide gel electrophoresis that the substrate was successfully purified and that thrombin cleavage of the purified substrate changes its FRET efficiency by monitoring its spectrum of emission.
To determine whether the FRET-based thrombin substrate can form interactions with thrombin's anion binding exosites and assess its potential for in vivo use, we measured the kinetics parameters of the substrate for thrombin in the presence of exosite inhibitors and thrombin's exosite variants as well as mouse thrombin and plasmin. We then compared the resulting kinetics parameters with commercially available thrombin substrates S2238 and technothrombin. We show that unlike S2238 and technothrombin, our FRET-based thrombin sensor forms strong interactions with thrombin's anion binding exosite I and displays preference for free thrombin compared to thrombomodulin (TM) bound thrombin which implied its preference for procoagulant thrombin rather than anticoagulant TM bound thrombin that activates protein C. Furthermore, our FRET-based thrombin substrate has potential to be used in mouse models because it can be efficiently cleaved by mouse thrombin and is relatively plasmin resistant.
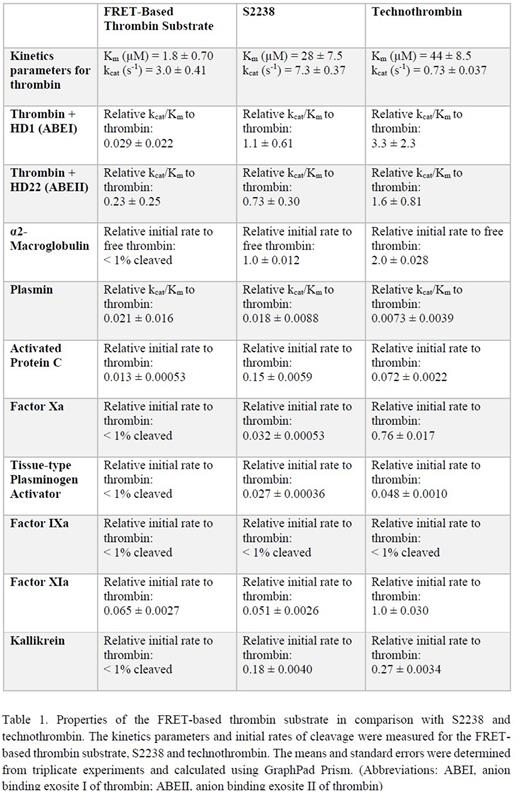
To assess the specificity of the FRET-based thrombin substrate for thrombin, we compared the rate of hydrolysis of the substrate by thrombin and several other enzymes in circulation and compared the results to S2238 and technothrombin. Our results indicated that when compared to commercially available thrombin substrates, our FRET-based thrombin substrate is highly specific for thrombin. Since the thrombin inhibitor α2-M is present in circulation, we also compared the rate of hydrolysis of the 3 substrates for α2-M bound thrombin and free thrombin. Unlike S2238 and technothrombin, our FRET-based thrombin substrate is uncleaved by α2-M bound thrombin. The key properties of the FRET-based thrombin substrate in comparison to S22238 and technothrombin are summarized in Table 1.
We then measured thrombin generation in plasma using our FRET-based thrombin substrate and showed that it is sensitive to direct oral anticoagulants in ways that are consistent with literature findings where thrombin generation was measured using technothrombin (Sivaraja et al. Plos One 2018 & Douxfils et al. Thromb Res 2012). We also confirmed that the FRET-based thrombin substrate itself is not a coagulation inhibitor since its presence did not alter thrombin generation parameters measured using technothrombin. Lastly, we confirmed that the FRET-based thrombin substrate can measure thrombin generation in whole blood since it successfully produced thrombin generation curves.
Disclosures
Gross:Bayer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees; LeoPharma: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Meyers-Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal